Drying or curing of dyes, printing inks, coatings and adhesives using ultraviolet radiation (UV) is a technology whose use is increasing in many areas of application.
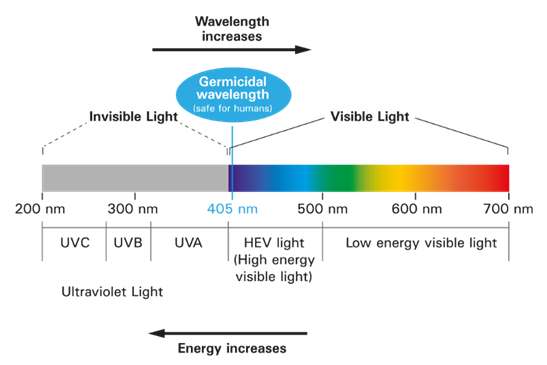
Ultraviolet radiation is the strongest form of radiation which occurs naturally in nature. It can be divided into following wavelengths:
UVC: 180-280nm
UVB: 280-320nm
UVA: 320-400nm
Curing occurs because of the photochemical reaction triggered by UV radiation, whereby a solid film is generated by crosslinking or polymerization.
Substances which can be cured by UV radiation are specially manufactured reactive liquids i.e. no solvents are required, hence resulting in an ecofriendly coating system. However photosensitive additives (so called photo initiators) are necessary.
.
Photoinitiators can be a problem when used with products used in the open air, because they often cause yellowing after a period of time.
.
Advantages of UV Curing
- No thermal energy required
- Faster conversion with high production speeds
- Substrate is not exposed to heat
- Results in good surface quality
- Dry film thickness is same as the application thickness
Medium pressure mercury lamps are the main kinds of UV lamps used. Because of their compact construction, UV curing systems have small carbon footprint. No exhaust air line and no cooling zones are required.
.
Since the UV coating liquids are highly reactive, they must be protected against inadvertent UV radiation during storage i.e., must not come in contact with sunlight.
.
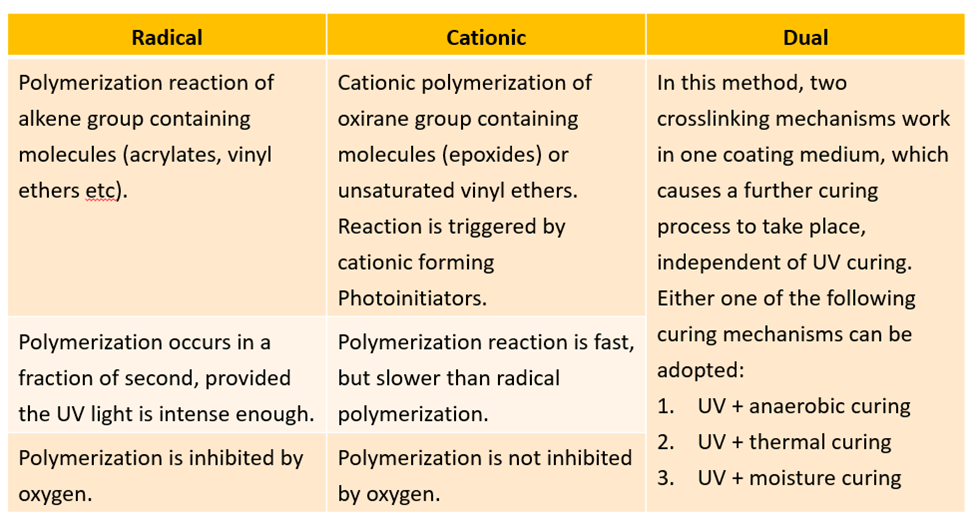
Curing Variations
There are 3 types of UV curing variations, which are well described and differentiated in the table below.
Oxygen Inhibition: Oxygen tends to react with the unsaturated compounds (alkene group containing molecules), preventing complete polymerization – this leads to cured deeper layers but uncured and sticky top layers. Thereby not providing the scratch or chemical resistance as per expectations.
.
.
EB Curing Process and Technology
Electron Beam (EB) curing is one of the most economically modern curing methods for polymerizing systems. EB are used to crosslink acrylate coatings on paper, PET, polyolefins, PVC, wood and metal.
Electron beams can penetrate a monomer containing, still liquid lacquer layer, and polymerize fully to create resistant layers. Regardless of the pigments or other extenders, one achieves very well cured coatings, as long as the lacquer coat thickness does not exceed the usable range of the electrons. The depth of penetration of electrons in the lacquer coat can be calculated and determined using more precise curves (electron crosslinking sheet). Drying and curing takes place in a matter of seconds.
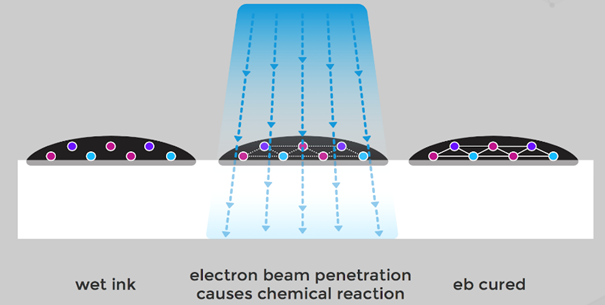
The electron beam accelerator is constructed analogous to cathode ray tube. Electrons are emitted in a vacuum by hot cathode, accelerated in electric field, redirected by magnetic field in the direction of anode, and meet on the surface to be irradiated that is being transported through the beam curtain at different speeds. When the electrons meet the surface, the short chain molecules react to long chain polymers and thus cure the surface.
.
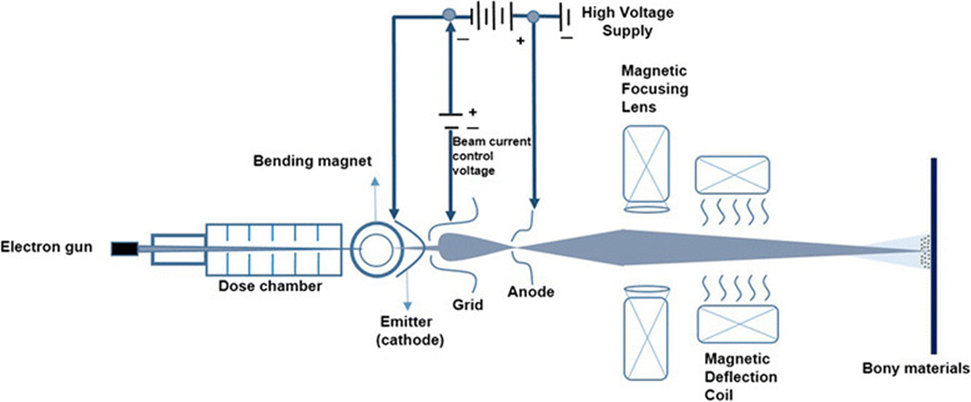
.
Advantages of EB Curing
- Ecofriendly process because of solvent free coating substances
- Curing of thick lacquer coatings in fraction of seconds
- Results in coatings with high crosslinking density
- No long wait times after drying – quick finishing
.
Disadvantages of EB Curing
- Polymerization is strongly hindered by oxygen
- High acquisition costs
.
What is the difference between UV & EB Curing Technology?
1. Difference in energy content
The smallest “bit” of EB energy is the electron. Unlike photons (in case of UV), electrons have mass, and they are negatively charged. The energy of electrons is determined by the voltage potential used in the acceleration process. Simple conversion of energy units shows that a 350nm UV photon is equivalent to 3.5 eV. This contrasts with 70,000 eV of electrons when they hit the surface. This means that EB electrons are on the order of 20,000 times more energetic than UV photons! If one considers that a typical C-H or C-C bond energy is on the order of 4 to 5 eV, then it becomes clear that EB has more than enough energy to break chemical bonds. This bond-breaking ability qualifies EB as a form of “ionizing radiation” as compared to UV, which is “non-ionizing.”
The bond-breaking power of EB is why free radical curing occurs without an added photoinitiator. The radicals can form directly by EB acting on the monomer and oligomer portion of the compositions.
.
2. Curing mechanism or Energy deposition mechanism
UV energy deposition is determined by the optical density of the material. UV curing can occur quite deep (multiple inches) into clear materials where there is limited absorbance of the base material in competition with the photoinitiators. However, UV curing has its limitations for pigmented coatings since they have high optical density and result in higher absorbance.
EB energy deposition does not depend on the optical density of the material. It penetrates equally well into clear and opaque materials.
.
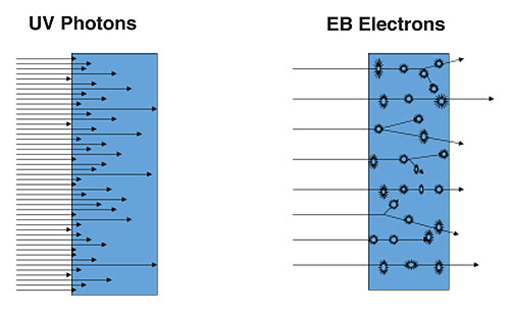
With UV, there are a large number of relatively low energy photons. The energy of the photons is concentrated at the surface and decreases exponentially though the material as predicted by Bouguer-Lambert Law. Longer wavelength photons may be less strongly absorbed and have enough penetration to cure through the material. With EB, there is a smaller number of higher energy electrons. Each electron may result in multiple initiating events. Given a selection of accelerating voltage appropriate for the material, the EB energy can be very uniform from the front to back surface of the material.
.
References
- Coating substrates and Textiles. Springer. Andreas Giessmann. 2020.
- Electron beam effect on biomaterials I: Focusing on bone graft materials. Biomaterials research. Soung Min Kim et al. 2015.
- EB curable Ink. Kao Collins Inc.
- UV+EB Technology. Q&A: EB Curing Technology. Stephen Lapin. 2015.