Corrosion is a type of oxidation that occurs when a material loses electrons to oxygen molecules in the surrounding air or water. In the context of industrial manufacturing, this material is typically a metal such as carbon steel or stainless steel.
.
Important note: Stainless steel has high corrosion resistance but can show signs of corrosion when exposed to extreme conditions. Also, there are 150 types of stainless steel with different alloy and chromium compositions, which causes the variation in corrosion resistance.
.
Oxidation occurs when atoms on the surface of a material (usually metal) lose electrons to oxygen in the surrounding air or water. The oxygen molecules gain electrons, forming an “oxide layer” with the metal. This thin layer can provide some degree of natural protection against further reactions, but damage has already been done. Oxidation weakens materials, making them more prone to breaks and fractures if exposed to additional stress.
.
.
Corrosion under insulation (CUI) refers to oxidation on the surface of an insulated metal pipe or vessel. The typical purpose of the insulation is to keep a hot asset from transferring heat. The side effect of the insulation is that it provides space between the high temperatures of an asset and the environment. In that space, oxygen and trapped moisture can meet and cause corrosion.
The estimated cost of corrosion is $2.2 trillion worldwide each year, which is 3% of the world’s GDP. The estimated cost of CUI is 40% – 60% of pipe maintenance costs. Now that we’re all on the same page with the costs of CUI, let’s dig a little deeper into the detection and prevention of CUI.
.
CUI of Carbon Steel
Carbon steel does not corrode simply because it is covered with insulation, but because it is contacted by aerated water. In a corroded system, insulation can provide an annular space or crevice for retaining water with full access to oxygen (air) and other corrosive media. If care is not taken, it can act as a wick or absorbent material and contribute contaminants that boost the corrosion rate. In carbon steel, the latter is mainly controlled by the temperature of the steel surface, availability of oxygen and water, and corrosive water-contaminants.
Contamination Sources:

Two primary water sources are involved in CUI of carbon steel. The first is breaks in the weatherproofing that lead to infiltration of external water sources to the metal surface, e.g., rainfall, drift from cooling towers, condensate from cold service equipment, steam discharge, and condensation on cold surfaces after vapor-barrier damage. The second is major corrosion wherever temperatures cycle from below the dew point to above-ambient temperatures. Here, the classic wet/dry cycle occurs when the cold metal develops water condensation that is then baked off during the hot/dry cycle. The transition from one to the other includes a period of damp/ warm conditions with attendant high corrosion rates.
Chlorides and sulfates are the main contaminants under insulation and can leach from the insulation or from external waterborne or airborne sources. They are particularly detrimental because their metal salts readily dissolve in water to yield highly conductive solutions. Furthermore, hydrolysis of the metal salts can create acidic conditions that lead to localized corrosion.
.
It is generally accepted that carbon steel at -4 to 149 °C is most at risk from CUI. Equipment operating continuously below -4 °C usually stays corrosion-free. Corrosion is reduced above 149 °C because the surface essentially stays dry. It tends to occur wherever water enters the insulation system when the temperature is below 149 °C and equipment is idle.
.
CUI of Stainless Steel
CUI in austenitic stainless steel is manifested by chloride-induced stress corrosion cracking (CISCC), also known as external stress corrosion cracking (ESCC) because the chloride source is external to the process environment.
The propensity for ESCC is known to be greatest when the following are present:
- Residual/applied surface tensile stresses
- Chlorides, bromide, fluoride ions
- Metal in the range 50 to 150 °C
- Electrolyte (water)
.
The stainless steels commonly affected by ESCC in the chemical process industries are the 300 series, 304 type, 316 type, 317L, 321, and 347. Other types can also undergo ESCC in certain conditions.
.
Effect of Stress: For ESCC to develop, enough tensile stress must be present in the material. If this is eliminated or greatly reduced, cracking will not occur. The threshold stress depends somewhat on the severity of the cracking medium. Most mill products, such as sheet, plate, pipe, and tubing, contain enough residual tensile stress to crack without external stress.
Effect of Chlorides: Chloride damages the passive protective layer on 18-8 stainless steels. Once the layer is penetrated, localized corrosion cells become active. Sodium chloride, due to its high solubility and ubiquity, is the most common corrosive species. While this neutral salt is the most common, it is not the most aggressive. Chloride salts of the weak bases and light metals, such as lithium, magnesium, and aluminum, can crack the 18-8 stainless steels even more rapidly in the right temperature and moisture conditions.
Effect of Temperature: The chief factor governing chloride concentration is the temperature of the metal surface. Heat has a dual effect: 1) Elevated temperatures cause evaporation from the surface and thus chloride concentration; and 2) As the temperature rises, susceptibility to ESCC initiation and propagation increases. ESCC occurs more often at 50 – 150 °C. Below 50 °C, chlorides do not concentrate to levels that cause ESCC. Above 150 °C, surface water is usually absent, and failures are rare.
.
Preventing CUI
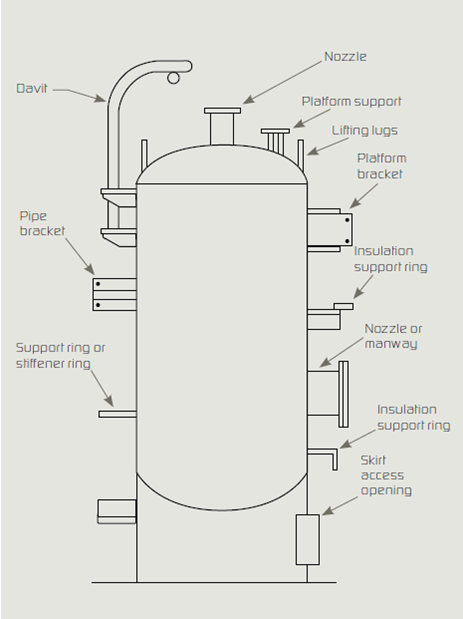
Attachments to vessels and piping stems are common locations where water bypasses the insulation and concentrates at the attachment point. Examples are shown in the Figure below. Attention to such details is key to a high-quality insulation system.
.
Prevention methodologies based on the design of the insulation system alone are not advisable or practical in a chemical plant. The physical properties of thermal insulation materials can vary widely. Some contain a leachable inhibitor to neutralize the pH of the water contacting the metal surface. The degree of water absorbency can also vary.
In some systems, the coefficient of thermal expansion influences the design: cellular glass expands almost as much as carbon steel, whereas cellular foam expands nine times as much and so requires expansion joints.
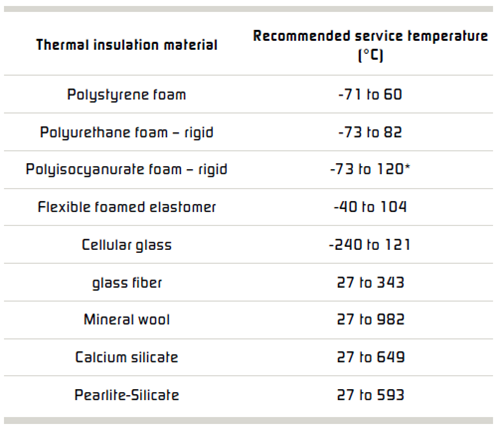
General industry experience suggests that corrosion can occur under all types of insulation. Common types of insulation materials and recommended service temperatures are listed in the Table above. Selecting and specifying the correct insulation material can reduce corrosion of both carbon and stainless steels.
.
For an insulation system to be considered reliable the maintenance cost and inspection costs should be eliminated. This is done by using life cycle cost analysis and the use of good CUI prevention tools.
.
- Thermal spray aluminum coating (TSA)
For services too severe for organic coatings, such as temperature cycling above and below 149 °C, TSA offers the best protection against CUI. It acts as a barrier coating and sacrificial anode, protecting the substrate at the sites of any chips or breaks in the coating.
- Replace personnel protection insulation with wire cages.
Thermal insulation is widely used to protect personnel from hot surfaces. This is unnecessary and creates a location for potential corrosion. Wire “standoff” cages are preferable because their simple design and low cost eliminate concerns with CUI.
- Stainless steel for small diameter pipe instead of carbon steel
.
Insulation properties important to reduce CUI:
- Low permeability
- Protection against water intrusion and retention
- Thermal expansion properties should be similar to carbon steel and stainless steel to reduce seal breakage.
- Consistent thermal properties – avoid products whose insulation values change with age; this can lead to dew point issues.
.
References
- How to prevent Corrosion Under Insulation. Eoncoat. Kim Feth.
- What is Corrosion Under Insulation? Concept Insulon.
- Corrosion Under Insulation. European Coatings Journal. Andreas Hoyer. 2020.

