
Solvent borne formulations, with their naturally lower surface tensions, wet readily and transfer well onto most substrates. The increasing shift to waterborne formulations, due to environmental concerns, has inherent problems of surface wetting, foaming, and flow & leveling, common to all waterborne systems. Waterborne systems require alcohols and surfactants to lower their surface tensions to acceptable levels for transfer, spreading and adhesion.
..
.
What is Dynamic Surface Tension?
Dynamic surface tension is a non-linear function of surface age and concentration. This must be considered when comparing surfactants. A coating application is a dynamic process, and active surfactants cause surface tensions to change as speeds and formulations change. The surface tension of coatings must be lower than the wetting tension of substrates to attain good laydown.
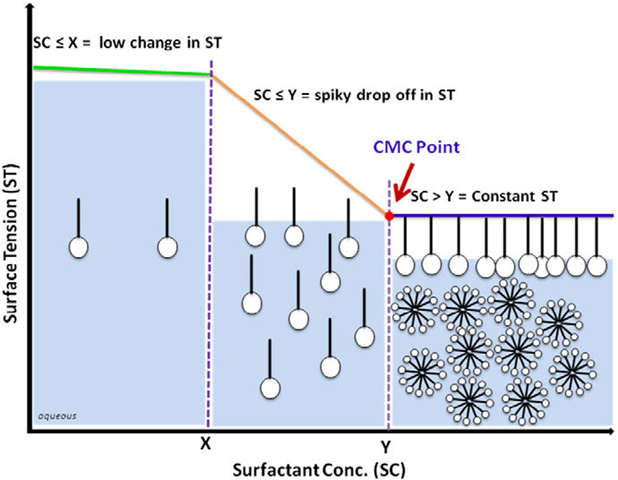
Classical belief is that many surface tension related properties such as detergency, foaming, and wetting, either maximize or minimize at the surfactant critical micelle concentration (CMC). These relationships, however, are based on classical measurements limited to static surface tension conditions, when equilibrium has been established between the surface layer and the bulk solution.
Dynamic surface tension measurements of active surfactants reveal levels at which surfactant effectiveness is at its highest, which is not necessarily related to equilibrium CMCs. Dynamic measurements more accurately reflect actual, in-process, surfactant, and coating performance. In effect, if you limit surfactant migration time (by using a faster coating process) you require more surfactant to perform the same job as in the slower process.

.
Surface Tension and Surfactants
Surface tension dictates whether a coating will wet and spread over or retract from a solid substrate. Coatings exhibit both an adhesive force that is a measurement of the degree of association of the coating for the substrate, and a cohesive force that is a measure of the degree of self-adhesion of the coating.
.
Spreading coefficient is the difference between work of adhesion and work of cohesion.
If work of adhesion > work of cohesion: Spontaneous spreading occurs.
If work of cohesion > work of adhesion: Retraction, a surface defect occurs.
.
Surfactants are used because of their ability to reduce surface tension. They are classified by the ionic charge of the surface acting part of the molecule. Anionic surfactants have a negative molecular charge, cationic positive, and nonionic no charge. Amphoteric have both positive and negative charges.
In general, surfactants with a smaller (lighter) molecule mass (short hydrophobic tail) diffuse more rapidly to the interface, and are vertically adsorbed at the interface, causing a compressive force to act on the surface thereby reducing surface energy or surface tension. Nonionic surfactants with ethylene oxide groups usually diffuse very rapidly to the surface while fluorinated surfactants are slower and more effective at equilibrium. Most surfactants at higher concentrations exert strong molecular attractions between adjacent molecules causing strong surface films, the strength of which determines the surface properties of the surfactant solutions.
.
.
.
Surfactant Performance
Surfactants that diffuse slowly may not lower surface tensions sufficiently, to acceptable levels and may be partially responsible for defects such as: Bénard cells, craters or pin holes, crawling or retraction, floating, orange peel, and picture framing (edge buildup).
Surfactants that diffuse rapidly can mitigate surface defects by eliminating surface tension gradients. This occurs through rapid surfactant migration from high concentrations (low surface tension) to low concentrations (high surface tension). This type of surfactant can reduce surface tension with the extra benefit of reducing or preventing foam. Formulators will sometimes mistakenly increase surfactant concentration to reduce gradients, rather than use a better surfactant.
Formulators should try to use a suitable surfactant that ideally has both low equilibrium and low dynamic surface tension values — low enough so that the coating is applied to the substrate at process speeds with a desirable viscosity.
.
Surface Energy
Transfer and spreading of coating on a substrate depend on the surface energy of the material delivering the coating, the surface tension of the coating, and the surface energy of the substrate receiving the coating. The substrate must have a surface energy higher than the coating, with forces of attraction great enough to promote good transfer and spreading, which in turn facilitates good adhesion.
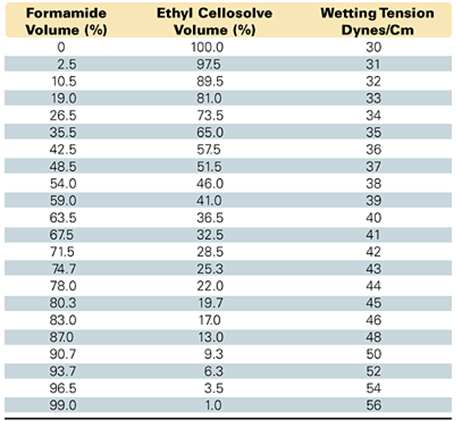
Measuring the wetting tension, or surface energy, of substrates is commonly and inexpensively done with the use of “dyne liquid” solutions. These are purchased as a series of premixed solutions, each of a given wetting tension in dynes/cm. This allows a series of wetting solutions to be formulated in a range from 30 to 56 dynes/cm, based on the proportions, as shown in the table. The dyne liquid solution (DLS) is used for measuring the wetting tension, or surface energy, of substrates. The dyne liquid solution (DLS) must readily wet the surface for the substrate material to be at a wetting tension equal to the surface tension of the DLS used.
.
.
References
- Paints & Coatings Industry. Dynamic Surface Tension and Surface Energy in Ink Formulation & Substrates. 2001.

