Polymeric colloidal emulsions are amongst the most popular choice of polymeric binder systems to be used in the inks, coatings, and adhesive industries. But still, there appears to be minimal source of scientific information available on this kind of chemistry.
In this Tech File article, we will try to cover and explain the binder chemistry, working mechanism, special & unique properties of the colloidal emulsions.
To begin decoding the subject, let us first try to understand the basic terms: “Colloid” and “Emulsion”.
Scientifically speaking, emulsion is a type of colloid. According to the original definitions, “A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance”; and “An emulsion is a mixture of two or more such liquids that are immiscible such as oil and water”. So that makes emulsion a “colloidal” solution of two liquids.
Four Types of Colloids: Sol, Emulsions, Foam and Aerosol
.
However, the above definitions would not be helpful for those studying or working with “polymeric emulsions”. Though the fundamentals of science remain the same, the approach and viewpoint keeps changing based on the topic of study.
With reference to polymeric emulsions, the term commonly associated with is “protective colloids”. Protective colloids are those “water-loving” colloid particles which remain solvated (hydrated) and preserve/enhance/protect the stability of polymer particles. Its because of these protective colloids that any added salt, ion or electrolyte cannot interfere with the polymer particle to affect its stability.
.
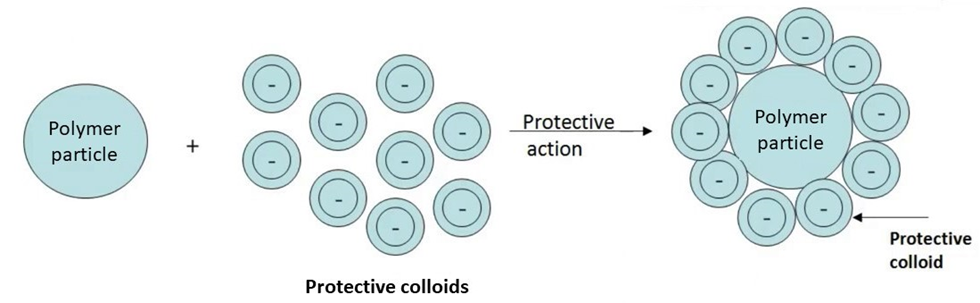
.
Properties of polymer emulsions depend not only on properties of polymers in the particles but also on stabilizers surrounding the particles. Stabilization of these latexes during polymerization and storage is of great importance. They can be stabilized by either steric, electrostatic or combination of these mechanism due to the existence of hydrophilic groups at the interface. Such groups can be introduced either by initiator residues, functional comonomers, or as in most of the practical cases by adsorbed surfactants and protective colloids (mechanism of stabilization of polymeric emulsions using emulsifiers/surfactants, has been covered in detail in the Tech File article: Basics of Emulsion Polymerization – Raw Materials and Mechanism. Hence is not being discussed in this article).
.
Examples of common Protective Colloids in Polymeric systems
- Polyvinyl alcohol (PVOH)
- Cellulosic derivates such as hydroxyethyl cellulose
- Colloidal silica
- Alkali soluble resin
- Starch
.
Stabilizing mechanism of Protective colloids
Polymeric protective colloids play an important role as steric stabilizer in emulsion polymerizations, where hydrophobic groups attach onto the polymer particles, while hydrophilic moieties stretch out in the water phase, thickening the hydration layer (as shown in the figure), to provide stability to the dispersion. Once electrolytes are added into the emulsion, they cannot act directly with the charges and emulsions on the surface of the latex particles due to the blocking function of protective colloid located on the periphery of the latex particles. Thus, protective colloid can effectively improve the stability of the emulsions against added electrolytes and ethanol. These protective colloids strongly affect not only the colloidal stability but also particle size, viscosity, and film properties of the latexes.
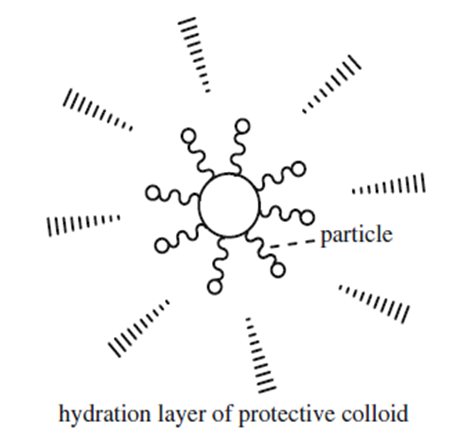
.
Partially hydrolyzed PVOH is preferred to obtain a small particle diameter, thixotropic flow, high viscosity stability even at low temperatures, but it has some disadvantages such as weak resistant properties of films against water, heat, and creep.
.
Colloidal Emulsions in Inks
With the increasingly stringent environmental requirements, most of the researchers attach importance to the water-based ink. The performance of water-based ink is determined by the ink binder, and polyacrylate emulsion has been major binder for inks, due to the superior properties in accordance with chemical resistance, film-forming ability, affinity to the pigment, and so on. However, polyacrylate emulsions show poor calcium ion stability and ethanol resistance.
The polyacrylate latex particles are encapsulated by the emulsifiers, and charges around the latex particle are in an equilibrium state. Therefore, the charges of electrolytes or additives will react with opposite charges on the surface of the latex particles, which can destroy the stabilizing layer outside the latex particles and cause imbalance between positive and negative charges. As a result, the emulsion becomes instable. On the other hand, ethanol is a de-emulsifier of polyacrylate emulsions because it can dissolve emulsifiers, which would lead to the loss of protective effect of the emulsifiers for the emulsions and cause the emulsion demulsification.
A research by Han et al demonstrates that the protective colloid can improve the polymerization stability, calcium ion stability, and ethanol resistance of the relative emulsions. Their research confirmed the stabilizing principle discussed above. It was observed that adding protective colloid in the formulation improved both calcium ion stability and ethanol resistance significantly (results of the experiment shared in the table below).
.
Another key property imparted by colloidal emulsions is the flat dilution curve for the binder i.e., addition of water to the emulsion does not drop down the viscosity of the emulsion significantly, which aids the formulators for developing economical ink formulations.
.
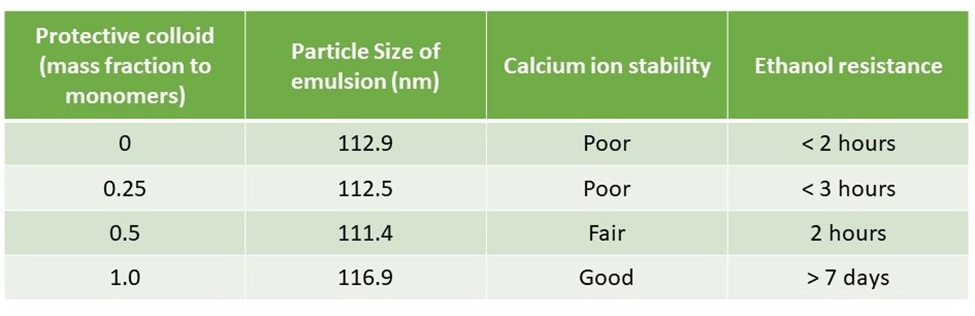
.
Colloidal Emulsions in Adhesives

Vinyl acetate (VAc) homopolymer and copolymer latexes have been widely used for adhesive and coating applications. PVOH has been used industrially as a dispersant in the synthesis of the copolymer emulsions of VAc. There are several reasons to adopt PVA in the commercial copolymer emulsions of VAc such as enhanced mechanical properties like tensile strength, Young’s modulus, and creep resistance. Low-molecular-weight emulsifiers such as sodium dodecyl sulfate are rarely utilized in above cases, although the emulsion polymerization using the emulsifiers proceeds easily.
.
Fully hydrolyzed PVOH form high water-resistant as well as heat-resistant, films due to its high crystallinity. However, the aqueous solution upon standing at low temperatures shows a drastic increase in viscosity. On the other hand, the viscosity of an aqueous solution of partially hydrolyzed PVOH does not change even at low temperatures, but resistant properties of the film against water and heat however are weak.
.
Although PVOH is mainly used in the emulsion polymerization of VAc, it is not employed in the emulsion polymerization of conjugated monomers such as styrene and acrylic monomers, due to the lack of stability during the polymerization. The poor stability has been believed to arise from the poor grafting ability of the conjugated monomers such as styrene and methacrylate. However, grafting ability of PVOH can be enhanced by introducing a thiol group in the PVOH molecule.
.
References
- A comparative study on water-based coatings prepared in the presence of oligomeric and conventional protective colloids. Process in Organic Coatings. Berber et al. 2011.
- The Effect of the Protective Colloid on the Property of Acrylic Emulsion. Han et al. 2016.
- Physical properties of acrylic copolymer emulsions using poly(vinyl alcohol) as a protective colloid in comparison with those using surfactants. Polymer International. Yuki et al. 2000.
- Study on the initial stage of emulsion polymerization of vinyl monomers using poly(vinyl alcohol) as a protective colloid – comparison between vinyl acetate (VAc) and methyl methacrylate (MMA). Progress in Colloidal Polymer Science. Suzuki et al. 2004.
- Preparation of polymer emulsions using a polyvinyl alcohol/ as protective colloid. Colloids and Surfaces. Nakamae et al. 1999.
- Sem 4 Physical Pharmaceutics 2. 1.4 Colloidal Dispersion Stability Protective Colloidal action. Pharmaplanet.

